Carbohydrates: Classification, Structure, and Function
Carbohydrates: Classification, Structure, and Function
Carbohydrates are one of the main classes of biomolecules essential for life. They primarily serve as a source of energy, but also play structural and regulatory roles in the body. Below is a comprehensive breakdown of the classification, structure, and functions of carbohydrates.
1. Classification of Carbohydrates
Carbohydrates are classified based on the number of sugar units (monomers) they contain. The main categories are:
a. Monosaccharides (Simple Sugars)
Monosaccharides are the simplest form of carbohydrates and cannot be hydrolyzed into simpler sugars. They are the building blocks of more complex carbohydrates.
- General Formula:
- Common Examples: Glucose, Fructose, Galactose
- Types of Monosaccharides:
- Aldoses: Have an aldehyde group (-CHO), such as glucose.
- Ketoses: Have a ketone group (C=O), such as fructose.
Monosaccharides can also be classified based on the number of carbon atoms:
- Trioses (3 carbon atoms)
- Tetroses (4 carbon atoms)
- Pentoses (5 carbon atoms) – e.g., ribose, deoxyribose.
- Hexoses (6 carbon atoms) – e.g., glucose, fructose.
b. Disaccharides
Disaccharides consist of two monosaccharide units linked by a glycosidic bond. These can be hydrolyzed into their constituent monosaccharides.
- Common Examples: Sucrose, Lactose, Maltose
- Sucrose: Glucose + Fructose (table sugar).
- Lactose: Glucose + Galactose (milk sugar).
- Maltose: Glucose + Glucose (malt sugar).
c. Oligosaccharides
Oligosaccharides consist of 3–10 monosaccharide units. These are found in some plant and bacterial cell walls and as parts of glycoproteins and glycolipids.
Based on the Number of Monosaccharide Units
Trisaccharides:
- These are oligosaccharides made up of three monosaccharide units.
- Example: Raffinose (composed of galactose, glucose, and fructose).
Tetrasaccharides:
- These are made up of four monosaccharide units.
- Example: Melezitose (found in some plant species).
Pentasaccharides:
- These contain five monosaccharide units.
- Example: Maltopentaose (found in starch).
Hexasaccharides:
- These consist of six monosaccharide units.
- Example: Maltopentaose (found in malt).
Heptasaccharides:
- Made up of seven monosaccharide units.
- Example: Maltodecaose.
Higher Oligosaccharides:
- Contain more than seven monosaccharide units (up to ten).
- Examples include some complex sugar derivatives, typically found in plants and microorganisms.
- Common Example: Raffinose (Galactose + Glucose + Fructose).
d. Polysaccharides (Complex Carbohydrates)
Polysaccharides are large, complex carbohydrates formed by more than 10 monosaccharide units, often hundreds or thousands. They serve as energy storage or structural components.
1. Based on the Type of Monosaccharide Units
Polysaccharides can be classified according to the type of monosaccharide that makes up their structure.
a. Homopolysaccharides
- These polysaccharides are composed of one type of monosaccharide repeated multiple times.
- Examples:
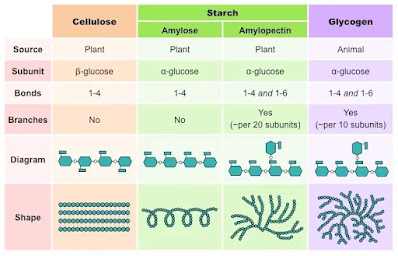
- Starch: A storage polysaccharide found in plants, made of repeating glucose units.
- Glycogen: The storage polysaccharide in animals, also composed of glucose.
- Cellulose: A structural polysaccharide in plants, made up of glucose units in a β-configuration.
- Chitin: A structural polysaccharide in the exoskeletons of arthropods, made of N-acetylglucosamine (GlcNAc) units.
b. Heteropolysaccharides
- These polysaccharides are composed of more than one type of monosaccharide.
- Examples:
- Hyaluronic acid: A glycosaminoglycan made of alternating units of glucuronic acid and N-acetylglucosamine, found in connective tissues.
- Pectin: A polysaccharide found in the cell walls of plants, composed of galacturonic acid and other sugars.
- Heparin: An anticoagulant polysaccharide composed of alternating disaccharide units, including glucosamine and glucuronic acid.
2. Structure of Carbohydrates
Carbohydrates have a general formula
Monosaccharides Structure
- Aldoses have an aldehyde group (-CHO) at the end of the molecule.
- Ketoses have a ketone group (C=O) within the molecule.
Monosaccharides can also form ring structures when the hydroxyl group (-OH) reacts with the aldehyde or ketone group. The ring can be in a pyranose (6-membered) or furanose (5-membered) form.
Glycosidic Bond in Disaccharides and Polysaccharides
- Glycosidic Bond: A covalent bond formed between two monosaccharide units through a dehydration reaction (release of water).
- The linkage can be:
- Alpha (α): If the hydroxyl group on the carbon-1 atom is below the plane of the ring (in glucose, it makes the structure less rigid).
- Beta (β): If the hydroxyl group is above the plane of the ring (as in cellulose, making the structure more rigid).
Polysaccharides Structure
- Starch: Composed of amylose (linear) and amylopectin (branched), both of which are made of glucose units connected by α-1,4 and α-1,6 glycosidic bonds.
- Glycogen: Similar to starch but with more frequent branching at α-1,6 glycosidic bonds, which allow for rapid breakdown when energy is needed.
- Cellulose: Composed of β-glucose units, which form long, straight chains that interact through hydrogen bonds to create a strong, rigid structure.
3. Functions of Carbohydrates
Carbohydrates serve various critical functions in living organisms. These include energy production, storage, and structural roles:
a. Energy Source
- Carbohydrates are the primary and preferred source of energy for the body, especially for the brain and muscles.
- Glucose is the key molecule used in cellular respiration to produce ATP, which powers most cellular activities.
- Carbohydrates undergo glycolysis, the citric acid cycle, and oxidative phosphorylation to generate energy.
b. Energy Storage
- Glycogen (in animals) and starch (in plants) are storage forms of glucose. They are stored in the liver and muscles in humans and animals, where they can be rapidly mobilized for energy when needed.
c. Structural Roles
- Cellulose (in plants) and chitin (in animals) provide structural support. For example, cellulose makes up the plant cell wall, providing rigidity and protection.
- In animals, glycosaminoglycans (e.g., hyaluronic acid) are involved in the formation of connective tissues, cartilage, and skin.
d. Cell Signaling and Recognition
- Carbohydrates are part of glycoproteins and glycolipids, which are essential for cell signaling, immune response, and cell-cell communication.
- Antibodies, which are proteins involved in immunity, often have carbohydrate chains attached that are involved in the recognition of pathogens.
e. Components of Nucleic Acids
- The sugar ribose (in RNA) and deoxyribose (in DNA) are essential components of nucleic acids. These sugars form the backbone of DNA and RNA molecules, linking nucleotides together.




Comments
Post a Comment