Principle of Chromatography:
Principle of Chromatography:
Chromatography is a laboratory technique used for the separation and analysis of mixtures. The principle of chromatography is based on the differential distribution of components of a mixture between two phases: the stationary phase and the mobile phase. The mixture is carried through the stationary phase by the mobile phase. Since the components of the mixture interact differently with the stationary phase and the mobile phase, they will travel at different rates, which results in their separation.
Stationary Phase: The stationary phase is the phase that remains fixed in place during the separation process. It can be solid or liquid, depending on the type of chromatography used.
Mobile Phase: The mobile phase is the phase that moves through or over the stationary phase, carrying the mixture with it. The mobile phase can be a liquid or gas.
The components of the mixture will interact differently with the stationary phase, causing them to move at different speeds, thus achieving separation. The separation is based on factors such as:
- Polarity: Molecules with different polarities interact differently with the phases.
- Size and Shape: Molecules of different sizes may be separated based on their ability to pass through the stationary phase.
- Affinities: Components might have different affinities for the stationary phase, which influences their retention time.
Types of Chromatography:
1. Paper Chromatography:
- Stationary Phase: Cellulose paper.
- Mobile Phase: A solvent (or a mixture of solvents) that moves through the paper by capillary action.
- Principle: The sample is applied to a paper strip, and the mobile phase (solvent) moves up the paper. The components of the sample separate due to differences in their solubility in the mobile phase and their interaction with the stationary phase.
- Applications: Used for the separation of pigments, amino acids, and other simple organic compounds.
- Procedure:
- Apply the sample to a paper strip.
- Place the bottom of the paper in a solvent.
- As the solvent rises up, components of the sample move with it at different rates.
- The separated components appear as distinct spots along the paper.
2. Thin Layer Chromatography (TLC):
- Stationary Phase: A thin layer of silica gel or alumina coated on a flat glass or plastic plate.
- Mobile Phase: A solvent (or mixture of solvents).
- Principle: The sample is applied to a TLC plate, and the mobile phase is allowed to move up the plate by capillary action. The components of the sample separate depending on their polarity and interaction with the stationary phase.
- Applications: Monitoring the progress of a reaction, identifying compounds, separating simple mixtures.
- Procedure:
- Apply the sample to a small spot on the TLC plate.
- Place the bottom of the plate in a solvent, and allow the solvent to travel up the plate by capillary action.
- Components of the sample move at different rates and separate along the plate.
- Visualize the spots by UV light or other detection methods.
3. Column Chromatography:
- Stationary Phase: A column packed with an adsorbent material (e.g., silica gel, alumina).
- Mobile Phase: A liquid solvent.
- Principle: The sample is applied at the top of a vertical column filled with stationary phase. The mobile phase is passed through the column, and as the sample moves down, its components separate based on their affinity for the stationary phase.
- Applications: Separation of larger quantities of compounds, such as in purifying chemicals or isolating specific biomolecules.
- Procedure:
- Load the sample into the top of the column.
- Pass the mobile phase through the column under gravity or pressure.
- Collect the separated components (fractions) from the bottom of the column.
- Analyze the fractions to identify separated compounds.
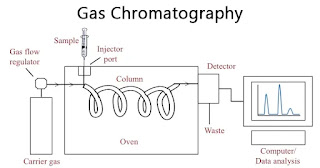
4. Gas Chromatography (GC):
- Stationary Phase: A liquid or solid adsorbent, often coated on a column.
- Mobile Phase: An inert gas (e.g., helium or nitrogen).
- Principle: The sample is vaporized and carried by the gas through a column. The components of the sample separate based on their interaction with the stationary phase, and their retention times are recorded as peaks.
- Applications: Analysis of volatile compounds, used in environmental, pharmaceutical, and food industries.
- Procedure:
- Inject the liquid sample into the column, where it vaporizes.
- The mobile phase (gas) carries the sample through the column.
- The components interact with the stationary phase, resulting in different retention times.
- The separated components are detected by a detector, and the results are plotted as a chromatogram.
5. High-Performance Liquid Chromatography (HPLC):
- Stationary Phase: A solid adsorbent material, typically packed in a column (e.g., silica gel).
- Mobile Phase: A liquid solvent (or a mixture of solvents).
- Principle: Similar to column chromatography, but the mobile phase is passed through the column at high pressure to achieve rapid separation. Components are separated based on their interaction with the stationary phase and elution times.
- Applications: Separation and quantification of complex mixtures (e.g., pharmaceuticals, proteins, environmental analysis).
- Procedure:
- Inject the sample into the column under high pressure.
- The mobile phase is pumped through the column at high pressure.
- The components are separated based on their affinity for the stationary phase.
- The components are detected and analyzed by detectors (e.g., UV, fluorescence).
6. Affinity Chromatography:
- Stationary Phase: A solid matrix (e.g., agarose or cellulose) with a specific ligand attached.
- Mobile Phase: A buffer solution.
- Principle: The stationary phase contains a ligand that specifically binds to the target molecule (e.g., enzyme, antibody). The mixture is passed through the column, and the target molecule binds to the ligand. Other components are washed away, and the target molecule is eluted by changing the conditions.
- Applications: Purification of specific proteins, nucleic acids, or antibodies.
- Procedure:
- Pack a column with the ligand attached to the stationary phase.
- Pass the sample through the column, allowing the target molecule to bind.
- Wash away non-target components.
- Elute the target molecule by altering the conditions (e.g., changing pH or salt concentration).
7. Ion Exchange Chromatography:
- Stationary Phase: A resin with charged functional groups (e.g., anion or cation exchangers).
- Mobile Phase: A buffer solution.
- Principle: Separation is based on the ionic charge of the components. Components with the same charge as the stationary phase will interact weakly, while those with the opposite charge will bind strongly.
- Applications: Purification of proteins, nucleic acids, or charged biomolecules.
- Procedure:
- Pack a column with ion-exchange resin.
- The sample is passed through the column, and components are separated based on their charge.
- Elute the components by gradually changing the ionic strength of the mobile phase.
Conclusion:
Chromatography is a versatile technique used in various scientific fields for separating, analyzing, and purifying compounds. The types of chromatography (such as paper chromatography, TLC, column chromatography, GC, HPLC, and others) offer different advantages depending on the nature of the sample and the level of separation required. The general procedure involves applying the sample, allowing it to separate due to different interactions with the stationary and mobile phases, and then analyzing the separated components.






Comments
Post a Comment